Chapter 3
Ch 3 - Prologue
Notes from 2014 CAP Congress / Congrès de l'ACP 2014
15-20 June 2014, Laurentian University / Université Laurentienne
Presentation By: CENDRIC, Matthew (University of Waterloo)
Observation of Spin Resonance Fields Occurring in Relativistic Velocity Particle Entanglement and Non-Causal Replication
Initial Experiment
Initial attempts at non-causal spin replication were performed using entangled pair photons streams, passing one stream through a super-cooled sodium cloud. The experiment produced minor and temporary variances in the resulting voltage output of the photon receptor. Similar effects were observed by accelerating an entangled pair electron stream to relativistic velocities in the TAPA particle accelerator. The most important result of the electron experiment being the production of a quantifiable variation in electron stream energy level.
The magnitude of the energy fluctuations in the electron stream can be described by the equation:
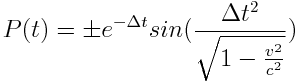
With a period of:
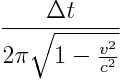
More interesting however is that when the electrons were passed through a polarization filter to control for spin, regardless of orientation, the relativistic or "delayed" entangled electron stream produced a net-negative energy integral described by:
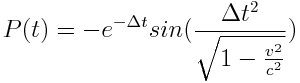
While the comparable non-relativistic electron stream showed the mirror net-positive energy integral described by:
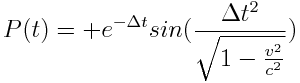
Increasing the power level of the particle accelerator and therefore increasing the relativistic particle velocity, we observed that the polarized power fluctuations, although equal and oppositely mirrored, were offset with respect the local frame of reference. This temporal offset was equal to the duration of the time dilation effect. In essence, the power fluctuations observed in the non-relativistic stream at time t0 were echoed by the relativistic stream at time t0+∆t.
Magnetic Resonance
Using the principles of Electron Paramagnetic Resonance spectroscopy, applying pulses of microwave frequency energy to the electron stream within a magnetic field allowed us to produce an EPR spectra graph by measuring the Hahn echo of the electron particles. Similarly we were able to observe the same echo emitted by the entangled relativistic electron stream at time t0+∆t. Increasing the irradiation frequency >;;; 285GHz and magnetic field strength of >;;; 10.2 T it became possible to produce extremely high-resolution spectra simply by measuring the resonance echo from the electrons.
The final question thus left to us was, would it work in reverse? Would it be possible to observe the resonance echo from the non-relativistic electron stream prior to applying the EPR measurement to its entangled partner?
This line of questioning led to an interesting conundrum. If it were possible to observe a non-causal resonance, could we induce a paradox by observing a resonance without inducing it? A simple binary, the essence of all digital information, being able to observe a result prior to the cause initiating it.
Non-Causal Replication
Testing the possibility of non-causal replication involves observing the EPR Hahn Echo at a moment in time prior to the EPR measurement being initiated on the entangled stream. Initial tests were conducted with too low of a particle accelerator beam power level, resulting in a time dilation ∆t value that was shorter than Hahn Echo T2 time producing an inconclusive result.
Subsequent experiments increasing the particle accelerator beam power level was able to produce sufficient ∆t to overcome the T2 duration, and conclusively detect the EPR resonance from the entangled electron stream prior to the initiation of the EPR scanner thereby achieving non-causal replication of magnetic resonance from the electron stream.
The tests conducted up until this point were attempting to produce that non-causal replication, without attempting to produce an observable paradox effect. Due to limitations with the current configuration of the particle accelerator, there is insufficient beam power to produce a ∆t large enough to make a causal determination to initiate the scanner or not after observing the existence or non-existence of a magnetic resonance. As a result of this limitation, to date we have been unable to intentionally force a paradox condition into being.
Efforts to use a randomization algorithm to non-deterministically generate the decision to initiate the EPR scanner were inconclusive. There was an observed 100% correlation between observer and generator, and every randomized experiment showed that a magnetic resonance was present when the EPR scanner was initiated, and no resonance was detected when it was not. This result strengthens the argument for a non-causal relationship, but the potential existence of a paradox remains elusive.
Future Experimentation
Currently TAPA has been powered down and is undergoing upgrades to increase its beam strength from 5.95 TeV to 9.8 TeV at which point testing is scheduled to resume in July 2015. To date the reason for our inability to induce a paradox is still unknown. Speculating on the problem leads us to two possible paths.
Either a paradox is impossible to create, regardless of the time dilation value the output from a system can not be obtained sufficiently far in advance to alter the input.
Or, the second possibility is that a paradox cannot be perceived by an observer that exists outside of the loop. That would imply that a paradox produces a closed time loop, and we may only be capable of perceiving the final effect of the loop once it has terminated in a manner that resolves the paradox.
Future experiments will be conducted with an aim to answer this question.
* * *
UN News Centre
with breaking news from the UN News Service
UN voices grief, pledges assistance after nuclear explosion strikes Canada
25 September 2015 - Secretary-General Won Soon-hee expressed grief and sorrow today and offered the full support of the United Nations after a massive nuclear explosion occurred in Canada, killing untold tens of thousands in the most densely populated part of the country.
"For the past seventy years, man has not been the author of death and destruction on the scale that has happened this week in Canada." Mrs. Won told reporters at UN Headquarters in New York this morning. "The world is stunned at the images we have been seeing over the past 24 hours, of a horror that we all wished to never again witness."
"I want to express my deepest sympathy and condolences to the Canadian people, and most especially to those who have lost family and friends. I grieve with you for your loss, and for the children who must now face a world transformed as I once did."
Mrs. Won said the UN would do all it could to provide humanitarian assistance as soon as possible. The UN Office for the Coordination of Humanitarian Affairs (OCHA) reported that its officials have already been dispatched to help with relief efforts. The UN has also alerted the International Search and Rescue Advisory Group (INSARAG), a network of countries and organizations dedicated to urban search and rescue and operation field coordination.
"Canada has long been at the forefront of international aid efforts, a generous benefactor to other countries in the wake of disaster and major crisis. Now it is time for the international community to stand with you in your time of need. I sincerely hope that the Canadian people and Government will be able to overcome this difficult time as soon as possible."
* * *
Experiment - Package 007
Institute of Temporal Physics (ITP)
Temporal Analysis Particle Accelerator (TAPA) Experimental Log
SEPTEMBER 23, 2015 - Peak Energy 9.8TeV Entanglement Resonance Receiver Test
...
11:24pm - Dr. Ridley extracts containment vessel, reports transmission received. Experiment cycle identifiers Zero-Zero-One through Zero-Zero-Seven are present and recorded.
Reminder: Cycle identifier Zero-Zero-Eght be transmitted at 8:32:16pm Apr 06, 2024.
11:49pm - Containment vessel recovered, contents are as follows.
Signed: Matthew Cendric, Alexandra Ridley
* * *
Experimental Package Item 004-A
Matthew Cendric, physicist and researcher, dies at 37
FEB 15, 2016
Waterloo physicist and leading researcher at the Institute of Temporal Physics, Matthew Cendric, PhD, died February 13th after a short battle with lung cancer. He was 37.
Cendric was dedicated to his life's work, working alongside his fellow researchers until the final weeks of his life. "The work was everything to him, he believed that what he was doing would change the world. And when you are there working with him, seeing his passion for what he was doing, you couldn't help be believe right along with him." said Riya Sastri, PhD, and junior researcher at ITP. Cendric had reportedly been offered a promotion to Institute Director on more than one occasion, but had refused each offer in order to continue to concentrate on his scientific work.
In a February 14 Facebook post, Alia Cendric-Lee, Cendric's sister wrote "Yesterday my brother Matthew passed away, only a few short months after being diagnosed with lung cancer that has spread to his brain. My brilliant and baffling brother. His head was forever in the clouds, seeing further than other men could see, but his heart was always at home. He was a good husband and father, and we will forever miss you. اللهم اغفر له".
Cendric was born in North York to immigrant parents, a Kurdish father and an Iranian mother who arrived from Turkey in 1977. He obtained his bachelor of Science in Physics at the University of Toronto in 1996 before travelling to England to study under the tutelage world-renowned physicist Stephen Hawking at the University of Cambridge's Department of Applied Mathematics and Theoretical Physics. He received his MSc with First-class honours in 1998 and graduated with a PhD in 2001. At Cambridge, he also met classmate Cheryl MacIver, whom he married in 1999.
During the summer of 2001 the two welcomed the birth of their first child, Jacob. In November of that year, Cendric returned to Canada with his wife and newborn son in tow amid the rapidly worsening political climate following the attacks in New York as well as a series of bombing attacks earlier in the year by the Real IRA, accepting a research position at the University of Waterloo. In the following years he authored 12 scientific publications before accepting a research position at the newly founded Institute of Temporal Physics in 2004. In 2005 they welcomed a second child, Mary, and then in 2008 a third, Fiona.
Cancer diagnosis
In September of 2015, just prior to one of Cendric's most important experiments and the culmination of eleven years of research, he collapsed suddenly. In October 2015, Cendric, who had never smoked, was diagnosed with stage-4 non-small-cell EGFR-positive lung cancer that had metastasized to the brain. "In retrospect" he wrote in January 2016 "I had been living with chronic headaches for some time, I had lost much of my appetite and had dropped close to 20 pounds, and had a lingering cough since the spring. The signs were there, but with the stress of the work I was doing and the long hours I was putting in, I had written them off as overwork and lack of sleep. It wasn't until my doctor recommended a CT scan after I fainted and hit my head that I thought there could be something wrong."
Despite being given a poor prognosis, his first reaction was a positive one. "He accepted the likelihood that he would die soon and it seemed to almost relax him. After the difficulty of the previous year, he seemed to take the news with relief."
Continuing to work
After his diagnosis he spent many hours researching cancer, how it spread, and reading up on new studies on ways to combat it. "He was very open about his disease." said PhD student Paul Grandy "He would talk about it to anyone who would listen, and spent hours talking to doctors and technicians about treatments, and studies, and how he could best contribute, as a subject, to the field of cancer research."
"He was an inspiration to us all." said Dr. Alexandra Ridley, a fellow leading researcher at ITP. "He had a fierce, driving energy in him that was most obvious when he was feeling his worst, a determination to ensure that something of value would be taken from his experience. That in the future others would reap the benefit."
Cendric is survived by his wife, Cheryl Eloise Cendric, CA, CPA, a financial advisor at Sun Life Financial; son, Jacob and daughters, Mary and Fiona; parents, Warhêl and Fatima; sister Alia Cendric-Lee; and Alia's wife Emily Cendric-Lee and their children Sophie and Zoe.
Cendric has chosen to donate his body to cancer research, and a memorial service will take place at 2 p.m. February 16 at the Waterloo Masjid at 213 Erb St W. (Those attending the memorial are advised to arrive before 1:30 p.m. to allow plenty of time for parking.)
The family has asked that donations be made to the Canadian Cancer Society in Cendric's memory in lieu of gifts or flowers.
* * *
Experimental Package Item 007-A
Phase IIB/III IG-11820 Immunotherapy Study in Subjects With Stage IIIB/IV Non-small Cell Lung Cancer (NSCLC)
Summary
This is a Phase IIb/III randomized, double-blind placebo-controlled study to assess overall survival and to compare the efficacy and safety of first-line therapy combined with Immugene immunotherapy IG-11820, Cisplatin + Bevacizumab/Avastin, or placebo in stage IIIB/IV non-small cell lung cancer (NSCLC).
IG-11820 is a suspension of recombinant Modified Vaccinia virus strain Ankara (MVA strain) carrying coded sequences for mouse MUG1 into cultured human lung cancer cell lines. IG-11820 is intended to provoke a strong immune system response in the patient, and to stimulate identification of cancer cells based on the shared abnormalities between IG-11820 and the patient's own cancer cells. The presence of foreign genetic material is intended to generate a hyperacute rejection of the patient's cancerous tumor.
Eligibility Criteria
Inclusion Criteria:
- Newly diagnosed and confirmed Stage IIIB/IV non-squamous non-small cell lung cancer [NSCLC]
- No more than 4 brain metastases, each ≤3 cm in size
- No evidence of cerebral edema
- Male or female at least 18 years of age and life expectancy of at least 3 months
- Male and female patients must agree to use at least one highly effective contraception or two effective contraceptive methods during the study period and for 3 months after the last treatment administration
- Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1
- Bilirubin (total) ≤1.5 x ULN
- Glomerular filtration rate ≥ 60 mL/min (according to modification of diet in renal disease [MDRD] formula or cockroft-gault formula)
- Lymphocytes count (total) ≥ 0.5x10E9/L
- Neutrophils ≥ 1.5x109/L
- Platelets count ≥ 100x10E9/L
- Serum alkaline phosphatase ≤ 2x ULN
- Serum transaminases (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) ≤ 1.5 x ULN
- White blood cells ≥ 3.0x10E9/L including hemoglobin ≥ 10.0 g/dL
Exclusion Criteria:
- Pregnancy or lactation
- Squamous carcinoma, or having presented a serious hemorrhage or recent hemoptysis
- Subjects with liver or bone metastases, spinal cord compression, or intractable back pain due to compressive or destructive mass
- Active or history of a known autoimmune disease
- Subjects under chronic treatment with systemic corticoids or other immunosuppressive drugs (eg. cyclosporine)
- Subjects who require emergent use of system steroids
- Known allergy to eggs, gentamicin, or platinum-containing compounds
Trial Details
Trial Status: Recruiting
Start Date: December, 2013
Expected Enrolment: 1024
Type of Trial: Treatment
Protocol IDs: NCT02857382, CA381-013
Trial Phase: Phase IIB/III
Medical Conditions: Non-squamous Non-small Cell Lung Cancer
Drugs: Cisplatin, Bevacizumab/Avastin
Lead Sponsor: Immugene Inc.
Type of Treatment: Biological: IG-11820
Principal Investigator(s): Patricia Castillo, MD
Date Posted: September 16, 2013
Last Updated: October 3, 2015
Centres / Contacts
The Ottawa Hospital
Patricia Castillo, MD
501 Smyth Road, Ottawa ON, K1H 8L6
613-737-7700
Princess Margaret Cancer Centre - Toronto ON
Randall Winsome, MD
610 University Avenue, Toronto ON, M5G 2M9
416-946-2000
Hamilton Health Sciences - Hamilton ON
Jane Darby, MD
699 Concession St, Hamilton ON, L8V 5C2
905-387-9495
* * *
Experimental Package Item 007-B
Immugene Announces Publication of Phase IIB/III Immunotherapy Study Results for Treatment of Non-squamous NSCLC
Ottawa, ON -- July 14, 2021 -- Immugene Inc. announced positive results today from the Phase IIB/III CA381-013 study of Ignerovax® showing clinically meaningful reduction in cancerous cells in non-squamous non-small cell lung cancer patients. The data is being published in the July 2021 issue of Cancer Research.
"Lung cancer is one of the deadliest and most common cancers, and one that has long resisted improvements in treatment and survivability over time. With these results we are seeing the first significant strides forward in improving the quality of treatments for lung cancer." said Patricia Castillo, Ph.D., director of the Ottawa Hospital lung cancer program. "Immunotherapy works much in the way of vaccines by training the body's immune system to recognize cancer cells as invaders by using foreign genetic material that it recognizes bonded with cancer cells and triggers a strong immune response that will attack cancer cells throughout the body."
About Non-Small Cell Lung Cancer (NSCLC)
Non-small cell lung cancer is the most common type of lung cancer. The three primary types of NSCLC are squamous cell carcinoma which is often linked to a history of smoking, adenocarcinoma which is the most common type of lung cancer found in non-smokers and younger people, and large cell carcinoma which grows and spreads quickly. Each year sees more than 1.35 million cases worldwide each year and accounts for 11% of total cancer diagnoses. It is the leading cause of cancer death worldwide in both men and women. About 60% of patients are first diagnosed with advanced NSCLC, meaning it has already spread beyond the lung. Median survival for all Stage IV NSCLC patients is 4 months from diagnosis and the 1-year and 5-year survival rates are 18.4% and 1.9% respectively.
About Ignerovax
Ignerovax® was recently granted Breakthrough Therapy Designation (BTD) by the U.S. Food and Drug Administration (FDA) for the treatment of non-squamous NSCLC. This designation was intended to expedite the approval of drugs intended to treat serious diseases that demonstrate substantial improvement over existing therapies.
CA381-013 (NCT02857382) is a Phase IIb/III multicenter study evaluating the efficacy and effectiveness of Ignerovax® over current phase 1 treatments and a placebo in conjunction with radiation therapy. The main study cohort included 1,024 patients of which 362 were assigned to the primary trial group, 347 to a group receiving a combination of Cisplatin + Bevacizumab/Avastin, and 315 to a placebo group. At the conclusion of the study showed a significant improvement in Median survival, as well as 1-year and 5-year survival rates over both the current standard for treatment and the control group.
Ignerovax Current Standard Control
Median Survival 11 months 4 months 3 months
1-year Survival 43.1% 19.0% 14.0%
5-year Survival 4.1% 2.0% 1.3%
About Immugene
Founded in 2009, Immugene is a leading oncology-focused biotech company based in Ottawa ON.
Posted: July 2021
A 12 page summary of detailed study results are attached to the press release.
* * *
Experimental Package Item 007-C
The following is hastily written on a scrap of paper and tucked within the pages of the summary of detailed study results.
Marius Pelkey, Age 52
Princess Margaret Cancer Centre Rm 613
Enrolled Oct 22, 2015
Group 1 ID IG11820-C164
61 Johnston Ave, Toronto
Died Nov 14, 2015 - cerebral hemorrhage
caused by aneurysm, unrelated to treatment
* * *
Experimental Package Item 007-D
Phase IIB/III IG-11820 Immunotherapy Study in Subjects With Stage IIIB/IV Non-small Cell Lung Cancer (NSCLC)
Patient Outcome Report
Study identifier: NCT02857382, CA381-013
Patient identifier: IG11820-B152
Initial diagnosis: Stage IV NSCLC Undifferentiated Carcinoma T2N1M1b with two brain metastases ≤ 3cm in size
Date of diagnosis: Oct 13, 2015
Study enrollment date: Oct 19, 2015
Patient-Reported Outcomes Measurements (PROMs) using FACIT Measurement System
FACT-G (Ver4) (27 questions, score 0-108)
FACT-Br (Ver4) (23 questions, score 0-92)
FACT-L (Vr4) (9 questions, score 0-36)
Week 0: 85 (26/25/12/21), 86, 34
Week 4: 81 (23/25/13/20), 79, 32
Week 8: 76 (20/24/12/20), 67, 29
Week 12: 58 (14/22/9/13), 48, 23
Week 16: 31 (5/19/4/3), 12, 8
Clinical Outcome
Treatment halted, final cycle completed Feb 9, 2015. Patient died after 16 weeks +4 days due to primary effects of brain metastases.
Patient Group
During this study, patient was assigned to Group III (control).
* * *
October 5th, 2015
MORTGAGE LOAN COMMITMENT
We are pleased to advise that, subject to our Solicitor being satisfied in all respects with the title to the property being offered as security, your application for a Fixed Rate mortgage has been approved as follows,
MORTGAGEE: RBC Royal Bank of Canada Ltd.
Address: 248 King St N, Waterloo, ON N2J 2Y7
Phone:
Contact Person: Melissa Dufresne
MORTGAGOR(S): ALEXANDRA RIDLEY
Address: 17 GREENBOUGH CT, KITCHENER, ON N2N 1L8, CANADA
Phone: 519 777 9352
Business Phone(s):
COVENANTOR(S) (If Any):
Address:
Phone:
Business Phone(s):
TYPE OF PROPERTY: RESIDENTIAL
PROPERTY TO BE MORTGAGED (include postal code) same as Mortgagor(s) address or:
17 GREENBOUGH CT, KITCHENER, ON N2N 1L8, CANADA
PAYMENT PROVISIONS
Principal Amount $ 130,000.00
Interest Rate 3.85 % per annum
Calculation Period Semi-annually not in advance
Interest Adjustment Date 15/10/16 (YY/MM/DD)
Payment Date and Period Semi-Monthly
First Payment Date 15/11/02 (YY/MM/DD)
Last Payment Date 25/10/16 (YY/MM/DD)
Amount of Each Payment (Principal & Interest) Dollars $ 438.03
Maturity Date 25/10/16 (YY/MM/DD)
Term: 120 months
Amortization: 180 months
Prepayment Provisions: SEE ATTACHED SCHEDULE 'A' and 'B'
Additional Provisions: FIXED RATE MORTGAGE EQUIVALENT RATE 3.809%
This commitment is null and void if not accepted by (Insert Date) OCTOBER 05/15, and if accepted by said date, may be cancelled or modified if the amount of the mortgage load is not fully advanced by (Insert Date) JANUARY 05/16. This commitment may not be assigned.
CONDITIONS OF ACCEPTANCE (Delete inapplicable conditions and have installed by Mortgagors)
1. TO BE PROVIDED BY MORTGAGOR:
Survey and Zoning Requirements
An up-to-date survey of this property by a Manitoba Land Surveyor, showing the improvements located entirely within the property lines and with no material encroachments. A zoning memorandum indicating complicance with by-laws as to yards and alignments. Such survey and zoning memorandum to be satisfactory to our Solicitor.
Realty Tax Certificate
A realty tax certificate confirming there are no outstanding taxes or such proof as is satisfactory to our Solicitor.
Taxes
Payment of realty taxes in advance by adding to each payment an amount equal to the annual taxes estimated by the Bank, divided by the number of payments for each year.
Declaration As To Possession
A signed declaration as to possession which will indicate occupancy of the encumbered property.
Order To Pay
A signed order to pay document which will detail how the proceeds of this mortgage loan will be disbursed.
Fire/Hazard Insurance
Insurance Policies with coverage for not less than the appraised value of the building(s) with (first/second) loss payable to us and with Standard Mortgage Clause attached must be delivered to our Solicitor.
Other
2. LEGAL AND APPRAISAL COSTS
You are to pay all legal fees, appraisal fees, and expenses incurred with respect to the mortgage loan and in compliance with the provisions outlined herein, whether or not the mortgage loan is advanced.
3. DISBURSEMENT OF LOAN PROCEEDS
The Bank will direct all funds in trust to our Solicitor to meet all charges, including retirement of prior indebtedness of any kind.
4. MORTGAGEE's SOLICITOR
The legal work on our behalf will be done by the Solicitor named in attached Schedule 'C'. You should deliver as soon as possible to our Solicitor: your duplicate title (if applicable); the survey and zoning documents referred to above; the first insurance policy; and the realty tax certificate.
The above is accepted and agreed to:
Alexandra Ridley
Date: October 5, 2015
RBC Royal Bank of Canada Ltd.
Per: Melissa Dufresne
Date: October 5, 2015
* * *
Stock Transaction Order Form
Toronto Stock Exchange: IMUG
Immugene, Inc.
Delayed quote: $42.07
Today's change: -1.41 (-3.35%)
P/E: Negative
Market cap: 1.15B
Open: $43.48
Previous close: $43.78
High: $43.61
Low: $41.77
Bid / Ask: $42.05 / $42.07
YTD % change: -7.03%
Volume: 49,103
Average volume (10-day): 42,432
Average volume (1-month): 48,038
Average volume (3-month): 47,398
52-week range: $39.85 to $56.35
Trailing P/E: Negative, not meaningful
Indicated annual dividend: $1.32
Dividend yield: 3.14%
Trailing EPS: -$0.31
Market Order BUY 3000 issued at 06/10/15 14:51:24.178
- BUY 100 $42.10 ($4,210.00) 06/10/15 14:51:24.254
- BUY 250 $42.15 ($10,537.50) 06/10/15 14:51:24.389
- BUY 1000 $42.25 ($42,250.00) 06/10/15 14:51:24.572
- BUY 50 $42.60 ($2,130.00) 06/10/15 14:51:25.933
- BUY 75 $42.75 ($3,206.25) 06/10/15 14:51:27.361
- BUY 1400 $43.50 ($60,900.00) 06/10/15 14:52:01.521
- BUY 125 $44.00 ($5,500.00) 06/10/15 14:52:02.424
Market Order BUY 3000 completed at 06/10/15 14:52:02.424 ($128,733.75)
* * *
INVOICE
Regal Uniform Service
84 Elm St,
Toronto, ON M5G 1H3
1-800-605-1992
GST #: 83750 2833 RT0483
DATE: Oct 19/2015
INVOICE #: 00004352
Ship To: Pick-up
210 Drumlin Circle, Unit #7
Concord, ON L4K 3E3
1-866-238-2451
P.O. #: 20009834
Sales Rep. Name: D. Makie
Ship Date: n/a
Ship Via: Pick-up
Terms:
Due Date: Nov 19/2015
Item # Description Price Qty Line Total
9000134 409/409 Men's Set - M - 2tone Postman Blu/Navy $48.95 1 $48.95
9002008 L407 - M - White $20.95 1 $20.95
9006003 Littmann Classic II Stethoscope: Grey 2203 $93.30 1 $93.30
Subtotal: $163.20
HST 13%: $21.22
Shipping & Handling: $0.00
TOTAL: $184.42
PAID: $184.42
TOTAL DUE: $0.00
***
01035384 10/14/15
PELKEY,MARIUS A
BD 11/08/63 M 75Y
BACHMAN,EMMANUELA H S403-02
3845-432-452-AM
Princess Margaret Cancer Centre
Toronto, ON
PROGRESS RECORD
DATE / NOTE PROGRESS OF CASE, COMPLICATIONS, CONSULTATIONS, CHANGES IN DIAGNOSIS, CONDITIONS OF DISCHARGE, INSTRUCTIONS TO PATIENT, ETC.
10/19/15 Neurology (cont)
A. Adenocarcinoma w/ paraneoplastic syndrome, clinically C/W limbic encephalopathy
P. No clearly beneficial treatment known and prognosis not good. Lung tumor cannot be eradicated, no other reasonable therapy. Disease is not reversible even if cancer was eradicated (which is not possible). Coping reasonably well for now, but cough and chest pain will likely grow worse. Palliative therapy and Hospice is indicated. Referral for psych assessment is recommended.
10/20/15 Psych
A. Subject meets DSM-5 criteria for major depressive disorder (MDD) with subthreshold manic symptoms including increased energy, increased speech, and inability to remain still. Classified as MDD with mixed features. DC 296.99 (F34.8) A-D, F, I w/ MDD A (1, 2, 5, 6, 8, 9), B, D - beyond expected norms for response to major illness - Sev. 296.32 (F33.1) with mixed features - A (3, 6), B, D - with melancholic features - A (2), B (1-3).
Prescription for Sertraline 50mg 1/d 30d. Must be monitored closely for possible exacerbation of manic symptoms or emergence of additional symptoms presenting into full hypomania episode.
P. Given a proscription for Zoloft - 1 tablet per day for 30 days. Any change ability to sleep, impulsive behaviour, wild swings in mood, or suicidal thoughts must be reported immediately. Scheduled for followup on 10/27.
10/21/15 A/P: Provided clinical trial information for three active studies currently enrolling at PMCC, patient to review.
10/22/15 A/P: Enrollment submitted for (NCT02857382, CA381-013) at patient and family's request.
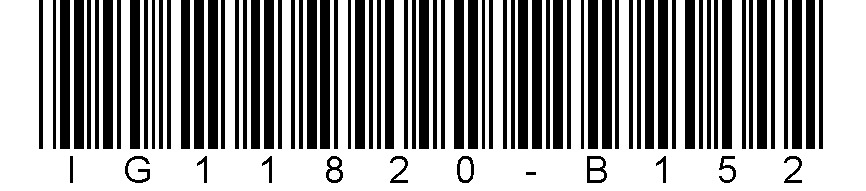
* * *
Atericorp Pharmaceuticals Inc.
Internal Memo
TO: Oncology Research Working Group
FROM: Themba Sibisi, VP of Oncology Research
DATE: October 28, 2015 11:32 AM EST
SUBJECT: Priority 1 Research Directive - MUG1 Immunotherapy Resource Redistribution
Effective immediately staff and resources will be reassigned to a new Priority 1 research project ATX-I-014. Redistribution will primarily impact pre-trial immunotherapy and recombinant gene therapy projects including ATX-I-013, 011, 008, and ATX-G-007, 005. Projects currently conducting clinical trials will be minimally impacted with the exception of ATX-I-009 which will be suspended indefinitely when Phase I trials end in 8 weeks.
ATX-I-014 will be responsible for developing a method to produce Ankara cultures carrying coded sequences for MUG1 (murinoglobulin 1 [Mus musculus]) into pancreatic adenocarcinoma, non-Hodgkins lymphoma, and leukaemia lines.
The position of Project Director will be filled by Dr. Miriam Bilodeau with initial development taking place in our Montreal lab. Once MUG1-Ankara synthesis has been completed, furthur research into individual cancer strains and clinical trial oversight will take place in Rochester MN, Detroit MI, and Hamilton ON. Individuals assigned to this project will be notified by Dr. Bilodeau by the end of the week.
We are playing catch up on this project, our competitors are already showing extremely promising results from Phase III clinical trials with their targetted treatment for lung and brain cancer. The purpose of this project is to apply the same techniques to our area of specialty where we have a golden opportunity to be first to market.
This priority directive has been approved by R.G. Gladston, President and CEO of Atericorp Pharmaceuticals Inc.
* * *
Ontario Ministry of Government Services / Office of the Registrar General
Medical Certificate of Death - Form 16
You must use the Stillbirth Registration Form 8 when registering stillbirths. This form must be completed by the attending physician, coroner, or designated person before a burial permit can be issued. Please PRINT clearly in blue or black in as it is a permanent legal record.
INFORMATION ABOUT THE DECEASED
1. Name of deceased (last, first, middle): Pelkey, Marius, Anthony
2. Date of death [month - by name, day, year (in full)]: March, 03, 2016
3. Sex (M or F): M
4. Age: 52
5. If under 1 yr. (months, days):
6. If under 1 day (hours, minutes):
7. Gestation age:
8. Birth weight:
9. Place of death (name of facility or location): Princess Margaret Cancer Centre (Hospital)
10. City, town, village or township: Toronto
11. CAUSE OF DEATH
Part I
Immediate cause of death / Approximate interval between onset & death
(a) Respiratory Arrest / hours
due to, or as a consequence of
Antecedent causes, if any, giving rise to the immediate cause (a) above, stating the underlying cause last
(b) Pneumonia / 2 months
due to, or as a consequence of
(c) Carcinoma of lung (primary) / 5 months
Part II
Other significant conditions contributing to the death but not causally related to the immediate cause (a) above
(a) Smoking 1ppd / 40 years
(b) COPD / 5 years
12. If decease was a female, did the death occur:
During pregnancy (including abortion and ectopic pregnancy):
Within 42 days thereafter:
Between 43 days and 1 year thereafter:
13. Was the deceased dead on arrival at the hospital?: No
14. Was there a surgical procedure within 28 days of death?: Yes
15. Date of surgery (mm/dd/yyyy): 02/09/2016
16. Reason for surgery and operative findings: Repair of internal injuries sustained by being stabbed, successfully completed with no complications
Autopsy particulars
17. Autopsy being held?: No
18. Does the cause of death stated above take account of autopsy findings?: No
19. May further information relating to the cause of death be available later?: No
Accidental or violent death (if applicable)
20. If accident, suicide, homicide or undetermined (specify):
21. Place of injury (e.g. home, farm, highway, etc.):
22. Date of injury (mm/dd/yyyy):
23. How did injury occur? (describe circumstances):
CERTIFICATION
By signing below, you certify that the information on this form is correct to the best of your knowledge.
24. Your signature (physician, coroner, RN(EC), other): X Emmanuela Bachman
25. Date (mm/dd/yyyy): 03/03/16
26. Your name (last, first, middle): Bachman, Emmanuela, Hene
27. Your title: Physician
28. Your address (street number and name, city, province, postal code): 108 Ewart Ave, York, ON, M6M 1M9
Personal information contained in this form is collected under the authority of the Vital Statistics Act, R.S.O. 1990, c.v.4 and will be used to register and record the births, still-births, deaths, marriages, additions or change of name, corrections or amendments, provide certified copies, extracts, certificates, search notices, photocopies and for statistical, research, medical, law enforcement, adoption and adoption disclosure purposes. Questions about this collection should be directed to the Deputy Registrar General at PO Box 4600, Thunder Bay ON P7B 6L8.
* * *
WATERLOO REGIONAL POLICE SERVICE
MISSING PERSON RISK ASSESSMENT TEMPLATE
Incident #: WM16102943
Year (yyyy): 2016
INSTRUCTIONS
This form must be completed by the investigating officer upon initial contact with the complainant. In the event the missing person returns prior to initial contact with the complaintant, this form is not required.
If any of the questions in the Risk Assessment are answered "YES", the incident requires immediate review and consultation with a supervisor to assess and allocate appropriate response and resources.
A supervisor must review all missing person investigations.
Ongoing monitoring of all missing person investigations is required. Other factors may need to be considered and documented when determining risk and investigative response.
RISK ASSESSMENT
Missing Person Name (Surname, Given Name(s)): CENDRIC, Jacob Tomas
Date of Birth (yyyy-mm-dd): 2001-05-19
Missing Person Information Questions
1. Is this person the subject of a crime in progress? E.g. abduction - NO
2. Is this person emotionally distraught, suicidal, or likely to cause harm to self or others? - YES
3. Has this person been involved in a violent or threatening incident prior to going missing? E.g. domestic - NO
4. Is this person vulnerable due to age, addition to drugs / alcohol, infirmity, inability to communicate, or other factors? - YES
5. Are there inclement weather conditions, terrain, inadequate clothing, or lack of proper equipment that would seriously increase risk to health? - NO
6. Does this person require essential medication? - NO
7. Does this person have any physical illness, disability, or mental health problems? - NO
8. Has this person been subject ot bullying / elder abuse? - YES
9. If previously missing, did he / she suffer any harm at that time? - N/A
10. Is this behaviour out of character? E.g. overdue and / or personal property has not been taken? - YES
11. Is this person scheduled to testify in court either as a witness or victim? - NO
12. Is this person involved in the sex trade, hitchhiking, gambling and / or transient lifestyle? - NO
13. Is this person associated to gang members or other organized crime? - NO
COMPLETED BY
Rank: Constable
Surname: Tham
Given Name: Sheng
Number: #4823
Signature: Tham Sheng
Date of signature (yyyy-mm-dd): 2016-03-14
REVIEWED BY (SUPERVISOR)
Rank: Sergeant
Surname: Trottier
Given Name: Marc
Number: #4498
Signature: Marc Trottier
Date of signature (yyyy-mm-dd): 2016-03-14
***
Corporate Carcinoma
Accident or Assassination? A Toronto VP of Research at a Montreal-based pharmaceutical company was killed in a car crash while in possession of confidential documents from a competitor. The person responsible for the crash is the son of a man who died while participating in a study run by that same competitor. Eye-360 investigates allegations of corporate espionage and links to organized crime.
Originally Broadcast on Saturday, April 2nd, 2016.
Voiceover: "Tonight, on Eye-360. A fatal car crash on the 401 exposes the dark underbelly of Big Pharma in Canada including corporate espionage, falsified research, and links to organized crime. Please stay tuned."
[Eye-360 introduction plays followed by a break for commercials]
Lauren Ashely: "Good evening. Tonight we cast our eye on pharmaceutical research in this country. It's a high stakes game that requires deep pockets, extraordinary patience, and a steely nerve to take big risks. The average cost to research and develop a new drug is nearly a billion dollars and takes 17 years from initial pre-clinical research to final government approval. But time and money aren't the only considerations, after spending half a decade in pre-clinical development fewer than 6% of new drugs ever make it through clinical trials. With so much riding on the outcome, can we really be surprised when someone looks for a shortcut?"
Lauren: "Monday March 7th, 2015. A fourteen car pileup westbound on the 401 near Allen Rd leaves two dead and ten injured. The two who died are Louisa Evans, age 39, a Sales Consultant with Johnson & Johnson and three months pregnant, and Themba Sibisi, Vice-President of Oncology Research at Montreal-based Atericorp Pharmaceuticals Inc. Witnesses attest that the vehicle driven by Mr. Sibisi was following closely behind that of the Ms. Evans in the right-most lane of the 401 Express where it merges with the collector lanes in advance of the Allen Rd. overpass. They say that a third vehicle approached from behind at a high rate of speed before swerving across three lanes of traffic, directly into the vehicle driven by Ms. Evans."
Lauren: "The impact forced Ms. Evans over far enough to collide head-on into the abutment where the Express and collector lane merge ends. Mr. Sibisi was unable to avoid the accident, colliding with Ms. Evans rear corner and the vehicle that caused the accident which proceeded to spin around and roll over. The driver of that vehicle was a man named Richard Pelkey, age 19, unemployed, never regained consciousness after the accident and later died in hospital."
Lauren: "During the course of the investigation, RCMP investigators discover confidential documents belonging to Immugene Inc. in the wreckage of Mr. Sibisi's vehicle. Immugene Inc. is a research company focusing on gene therapy treatments for cancer, and a direct competitor with Atericorp. The documents were preliminary results on a Phase III trial of a new drug developed by Immugene that showed very promising early results in treating lung cancer, doubling the survival rate of patients over existing treatments."
Lauren: "How did these documents get into Mr. Sibisi's possession? Why has the RCMP not pursued the investigation into possible corporate espionage against a competitor? Why has Immugene resorted to filing a lawsuit against Atericorp for patent infringement in what is potentially a criminal matter? More, coming up next."
[Break for commercials]
Voiceover: "To learn more about corporate espionage, we interviewed a lawyer for a medical research company. The person did not want to be identified on camera, so their face has been hidden and their voice has been disguised."
Lauren: "Can you tell me, how common is spying among medical research companies?"
Person: "Well, I don't know if it's common, but it happens. It's a competitive business, being the first to market with a new drug is worth billions of dollars. So research, especially preliminary work is very carefully guarded. Early clinical results from a competitor for a drug that is promising can save a company five or ten years of work and hundreds of millions of dollars. It's a surprise that you don't hear about it happening more often, really."
Lauren: "But, if the impact is so significant, why would the RCMP decide not to investigate?"
Person: "Because if it's one Canadian company stealing from another, the politicians really don't care. If one or the other benefits, the money is still coming into Canada so why does it matter? The public doesn't have much sympathy when you are talking about multi-billion dollar companies, and a politician isn't going to win much in the way of popular opinion if they promise to crack down on corporate espionage. If it was a foreign company then that's a different matter. If, say, it was China coming in and stealing secrets from Canadians then the RCMP and CSIS would be all over it. But if it's local, they don't give a rat's ass. It's up to a company to defend themselves in court."
[Back to studio]
Lauren: "Our investigators spent some time digging into Atericorp's finances, and they uncovered something interesting. Although corporate donations are banned in municipal politics in Montreal, in 2001, 2005, and 2009, 9 out of 12 of Atericorp's board members and every senior executive in the company donated the maximum allowable contribution for individuals to the municipal political party Union Montréal. Each of the three elections saw Union mayoral candidate Gérald Tremblay win. Tremblay served as mayor of the city until November 2012 when he resigned from politics on the heels of the Charbonneau Commission which was investigating potential corruption in the management of public construction contracts in Quebec. During one hearing, it was alleged that Union Montréal received a percentage of a sewerage rehabilitation contract awarded by the City of Montreal to a mafia-linked cartel. Further allegations against the Mayor included linking him to illegal financing with the mafia."
[Break for commercials]
Lauren: "Whether or not Atericorp has connections to organized crime isn't yet clear, but why did the crash happen in the first place? Was it really an accident, or was it intentional? And who is Richard Pelkey? Eyewitness accounts lead us to believe that the crash resulting from reckless driving was far from unintentional."
Lauren: "As it turns out, Richard Pelkey did have a connection to Themba Sibisi and Atericorp, though an indirect one. During the months prior to the accident, Richard's father, Marius Pelkey was suffering from terminal lung cancer and had been participating in a drug trial being run by Immugene, the very same drug trial that Mr. Sibisi had the preliminary results for in his car. In fact, on the day of the accident, Richard Pelkey had just finished burying his father who had passed away a few days before. Did Richard know who Mr. Sibisi was? Was he aware of the stolen study results? Did he intentionally crash his vehicle in order to kill Mr. Sibisi? Or was the whole thing a simple, poorly timed accident? Unfortunately, Richard Pelkey's true motivation will forever remain a mystery.
Lauren: "There is one final twist to this story. The study results at the heart of the whole affair are only a summary excerpt from what is presumed to be a larger document. The pages are not dated, but they do contain information reporting on the 1-year and 5-year survival rates of patients participating in the study, a standard measure of survivability and effectiveness of treatment. The twist is that the study itself commenced in December 2013, only 2 1/2 years ago. Which leads us to question Immugene's role in this. Is the study a fake? Is it being used to frame Atericorp? Or are they falsifying results?"
Lauren: "Thank you for joining us, I am Lauren Ashely and this has been Eye-360."
* * *
Post-Cancer Care Plan
This Care Plan will facilitate cancer care following active treatment. It includes important contact information, a treatment summary, recommendations for follow-up care testing, a directory of support services and resources, and other information.
Post-Cancer Care Plan for Lung Cancer
Prepared by: Amanda Miner on 04/07/16 at Grand River Regional Cancer Centre
GENERAL INFORMATION
Patient name: Matthew Cendric
Medical record number: 01433583
Phone (home): (226) 236-8826
Date of birth: 06/21/1978
Age at diagnosis: 37
Gender: Male
Support contact: Cheryl Cendric, (226) 236-7342
Care Team
Medical oncologist: Dr. Hurst, (519) 749-4300 ext. 3843
Primary care physician: Dr. Halabi, (519) 777-9352
Nurse/nurse practitioner: Amanda Miner, (519) 777-9352
Mental health/social worker: Nicholas Glass, (519) 635-7254
Surgeon: Dr. Forsythe, (519) 573-7763
BACKGROUND INFORMATION
Diagnosis: NSCLC Undifferentiated Carcinoma
Tobacco use-past: No
Tobacco use-current: No
Other heath concerns: Brain metastases, two tumors
Location: RUL (Right Upper Lobe)
Relevant preoperative findings: n/a
Definitive surgery: On 03/17/2016 of non-small cell carcinoma via Wedge resection
Completeness of resection: R1 (Microscopic residual tumor)
TNM stage: T2 (Tumors greater than 3 cm but less than 7cm), N1 (The tumor has spread to nearby nodes on the same side of the body), M1b (Distant metastasis (in extrathoracic organs))
Pathologic stage: IV (The tumor has spread to distant organs outside of the chest)
Final apthologic details: Undifferentiated
TREATMENT PLAN & SUMMARY
Patient's Height: 71 in
Pre-treatment
Patient's weight: 172 lb
Patient's BSA: 1.975 m^2
Patient's BMI: 24.1
ECOG performance status: 0 (Mildly symptomatic)
Post-treatment
Patient's weight: 164 lb
Patient's BSA: 1.936 m^2
Patient's BMI: 23.0
ECOG performance status: 1 (Restricted in physically strenuous activity, but ambulatory and able to carry out work of a light or sedentary nature, e.g. light house work, office work)
Regimen: Participant in IG-11820 Immunotherapy Study (CA381-013), patient treatment identifier: IG11820-C164
Treatment on clinical trial: Yes
Chemotherapy treatment period: 10/26/2015 - 02/15/2016
Major side effects of treatment: Nausea/vomiting, Neuropathy, Low blood count, Fatigue, Diarrhea, Pneumonia
Reason for stopping treatment: Completed therapy
Treatment-related hospitalization: Yes
Ongoing toxicities: Yes: Peripheral neuropathy
Radiation therapy: 60Gy administered 02/18/2016 - 03/31/2016
FOLLOW-UP CARE
Upon screening, the patient has been determined to have the following issue(s):
Patients - Please consult your health care provider for medical advice specific to you before using any medications, supplements, or other products, and before beginning any lifestyle program.
Needs, concerns / Suggested intervention(s)
Memory problems and/or confusion
Patients should know that 25% of cancer patients have cognitive dysfunction after treatment and it usually gets better over time
- Rule out depression, sleep disturbance
Wellness (e.g. diet, exercise, smoking cessation)
- Maintenance of body weight as weight gain is associated with recurrence
- Regular physical activity (e.g. walking 20 minutes daily)
- Avoidance of smoking/smoking cessation counseling, if appropriate
- Limitation of alcohol intake to less than 1 drink, 2-3x per week
Surveillance / When and how often / Coordinating provider
Medical oncology visits / Every 6-12 months for 2 years / Dr. Hurst
Imaging: Chest CT with contrast / Every 6-12 months for 2 years / Dr. Hurst
Imaging: Heat CT / Every 6-12 months for 2 years / Dr. Hurst
Colonoscopy / Every 5 years / Dr. Halabi
Referrals provided
Social worker: Nicholas Glass
POST-TREATMENT
It is very important to keep all follow-up appointments. During these visits, your doctors will ask about symptoms, do physical exams, and may order blood tests or imaging tests such as CT scans or x-rays. Follow-up is needed to check for cancer recurrence or spread, as well as possible side effects of certain treatments. Almost any cancer treatment can have side effects. Some may last for a few weeks to several months, and others may be permanent.
Managing Memory Changes
25% of people with cancer report memory and attention problems after chemotherapy which is often called "chemo brain". Most patients describe this as a fog, or having their head stuffed with wool, which can lead to problems with speech, memory formation, or attention. These effects can start soon after treatment, or may not appear until months later.
If you have memory and concentration problems, contact your doctor and ask about seeing a specialist (a neuropsychologist) to help you with these problems.
If you think you suffer from depression or anxiety, these problems can also affect your attention, concentration, and memory.
If you develop a feeling of pain (stabbing or burning) or persistent tingling, weakness, or numbness in your hands or other extremities, your doctor may proscribe medication to manage the symptoms of peripheral neuropathy.
 Garret Rempel
Garret Rempel